Focusing on innovation and patient accessibility, we have established a diversified and complementary R&D pipeline. Most products are independently developed through our own platform, and we have a number of potential novel antitumor targets drugs. Our products respond to a vast array of diseases, including cancer, autoimmune, metabolic, neurological, and infectious diseases.
As we continue to enrich our pipeline and explore combination treatments, our fields of innovation R&D has expanded from monoclonal antibodies to small molecule drugs, polypeptide drugs, antibody-drug conjugates (ADCs), bispecific antibody or multispecific antibody drugs, nucleotide drugs and etc. We will continue to further explore the next generation of innovative therapies for cancer and autoimmune diseases.
R&D Pipeline
- Oncology
- Autoimmune
- Metabolic
- Neurological
- Infectious
- Global Developed
-
JS001sc
PD-1
Tumors
A subcutaneous injection formulation developed by Junshi Biosciences on the basis of toripalimab
-
JS015
DKK1
Tumors
-
JS112
Aurora A
SCLC
-
JS003
PD-L1
Tumors
-
JS019
CD39
Tumors
A recombinant fully human anti-CD39 monoclonal antibody developed by Suzhou Kebo Ruijun owned by Junshi Biosciences and Beijing Enruini
-
JS113
EGFR 4th Gen
NSCLC
-
JS006(TAB006)
TIGIT
Tumors
A recombinant humanized anti-TIGIT monoclonal antibody developed independently by Junshi Biosciences
-
JS101
Pan-CDK
Breast cancer, etc.
-
JS116
KRAS
Tumors
-
JS007
CTLA-4
Lung cancer, melanoma
A recombinant humanized anti-CTLA-4 monoclonal antibody developed independently by Junshi Biosciences
-
JS105
PI3K-α
Breast cancer, renal cell cancer
An oral small molecule inhibitor targeting PI3K-α jointly developed by Junshi Biosciences and Risen Biosciences
-
JS201
PD-1+TGF-β
Tumors
-
JS009(TAB009)
CD112R/PVRIG
Tumors
A recombinant humanized monoclonal antibody against human CD112R developed independently by Junshi Biosciences
-
JS107
Claudin18.2 ADC
Gastrointestinal cancer
An antibody-drug conjugate (ADCs) targeting Claudin18.2 developed by Junshi Biosciences
-
JS203
CD3+CD20
Tumors
-
JS012
Claudin 18.2
Gastric cancer
-
JS108
TROP2 ADC
TNBC
-
JS103
Uricase
Hyperuricacidemia
-
JS014
IL-21
Tumors
A fusion protein consisting of recombinant IL-21 and nanobody against human serum albumin (HSA) co-developed by Junshi Biosciences and Anwita Biosciences
-
JS110
XPO1
Multiple myeloma, etc
A small molecule inhibitor of the nuclear export protein XPO1
-
UBP1213sc
BLyS
Systemic lupus erythematosus
-
JS111
EGFR exon 20
NSCLC
-
JS026
S protein
COVID-19
-
Tifcemalimab
BTLA
Lung cancer, Melanoma, etc
World’s first-in-human anti-BTLA mAb against tumor
-
JS005
IL-17A
Psoriatic, spondylitis
-
JS109
PARP
Ovarian cancer
-
Bevacizumab
VEGF
NSCLC
-
Ongericimab
PCSK9
Hyperlipidemia
Ongericimab is a recombinant humanized anti-PCSK9 monoclonal antibody independently developed by Junshi Biosciences for the treatment of primary hypercholesterolemia and mixed dyslipidemia
-
JT001/VV116
RdRp
COVID-19
VV116 is an oral nucleoside analog drug that can inhibit the replication of SARS-CoV-2
-
Toripalimab
PD-1
Tumors
-
Adalimumab
TNF-α
Rheumatoid Arthritis, etc
-
Etesevimab
S protein
COVID-19
Product Details
- Product name : TUOYI®
- Drug Code : Toripalimab
- Target : PD-1
- Equity : In-house
Toripalimab is an anti-PD-1 monoclonal antibody developed for its ability to block PD-1 interactions with its ligands, PD-L1 and PD-L2, and for enhanced receptor internalization (endocytosis function). Blocking PD-1 interactions with PD-L1 and PD-L2 promotes the immune system’s ability to attack and kill tumor cells.
More than thirty company-sponsored toripalimab clinical studies covering more than fifteen indications have been conducted globally by Junshi Biosciences, including in China, the United States, Southeast Asia, and European countries. Ongoing or completed pivotal clinical trials evaluating the safety and efficacy of toripalimab cover a broad range of tumor types including cancers of the lung, nasopharynx, esophagus, stomach, bladder, breast, liver, kidney and skin.
In China, toripalimab was the first domestic anti-PD-1 monoclonal antibody approved for marketing (approved in China as TUOYI®). Currently, there are six approved indications for toripalimab in China:
- 1. unresectable or metastatic melanoma after failure of standard systemic therapy;
- 2. in combination with cisplatin and gemcitabine as the first-line treatment for patients with locally recurrent or metastatic nasopharyngeal carcinoma (“NPC”);
- 3. recurrent or metastatic NPC after failure of at least two lines of prior systemic therapy;
- 4. locally advanced or metastatic urothelial carcinoma that failed platinum-containing chemotherapy or progressed within 12 months of neoadjuvant or adjuvant platinum-containing chemotherapy;
- 5. in combination with paclitaxel and cisplatin in first-line treatment of patients with unresectable locally advanced/recurrent or distant metastatic esophageal squamous cell carcinoma (“ESCC”);
- 6. in combination with pemetrexed and platinum as the first-line treatment in EGFR mutation-negative and ALK mutation-negative, unresectable, locally advanced or metastatic non-squamous non-small-cell lung cancer (“NSCLC”).
The first three indications have been included in the National Reimbursement Drug List (“NRDL”) (2021 Edition). Toripalimab is the only anti-PD-1 monoclonal antibody included in the NRDL for melanoma and NPC.
In the United States, the FDA is reviewing the Biologics License Application (“BLA”) resubmission for toripalimab in combination with gemcitabine and cisplatin as first-line treatment for patients with advanced recurrent or metastatic nasopharyngeal carcinoma (“NPC”) and for toripalimab monotherapy for the second-line or later treatment of recurrent or metastatic NPC after platinum-containing chemotherapy. The FDA has granted Breakthrough Therapy designations for toripalimab in combination with chemotherapy for the first-line treatment of recurrent or metastatic NPC as well as for toripalimab monotherapy in the second or third-line treatment of recurrent or metastatic NPC. Additionally, the FDA has granted Fast Track designation for toripalimab for the treatment of mucosal melanoma and Orphan Drug designations for the treatment of esophageal cancer, NPC, mucosal melanoma, soft tissue sarcoma, and small cell lung cancer (“SCLC”).
In the European Union, marketing authorization applications (MAA) were submitted to the European Medicines Agency (EMA) and the United Kingdom’s Medicines and Healthcare products Regulatory Agency (MHRA) in November 2022 for: 1) toripalimab combined with cisplatin and gemcitabine for the first-line treatment of patients with locally recurrent or metastatic NPC and 2) toripalimab combined with paclitaxel and cisplatin for the first-line treatment of patients with unresectable locally advanced/recurrent or metastatic ESCC. In December 2022, the EMA accepted the MAA.
Mechanism of Action
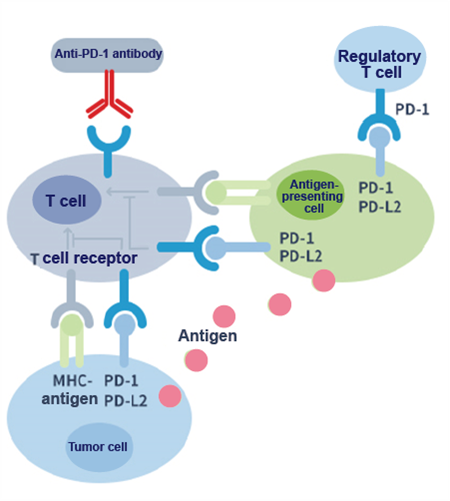
PD-1 is a T cell surface receptor; it is an immune checkpoint molecule in the co-inhibitory signal pathway of the T cell. As shown in the following figure, the binding of the PD-1 ligands, PD-L1 and PD-L2, to the PD-1 receptor found on T cells inhibits T cell proliferation and cytokine production. Upregulation of PD-1 ligands occurs in some tumors and signaling through this pathway can contribute to the inhibition of T-cell activation and immune surveillance of tumor cells. Anti-PD-1 monoclonal antibodies can block the PD-1/PD-L1 pathway and thereby restore the immune function of T cells.
Main research literature
- Serial number Journal name Document title Published on Link
- 1 Nature Medicine Toripalimab or placebo plus chemotherapy as first-line treatment in advanced nasopharyngeal carcinoma: a multicenter randomized phase 3 trial 2021 Open link
- 2 Signal Transduction and Targeted Therapy Toripalimab plus chemotherapy as second-line treatment in previously EGFRTKIs treated patients with EGFR-mutant advanced NSCLC: a multi-center phase II trial 2021 Open link
- 3 Journal of Clinical Oncology Efficacy, Safety, and Correlative Biomarkers of Toripalimab in Previously Treated Recurrent or Metastatic Nasopharyngeal Carcinoma: A Phase II Clinical Trial (POLARIS-02) 2021 Open link
- 4 JAMA Network Open Safety, Antitumor Activity, and Pharmacokinetics of Toripalimab, a Programmed Cell Death 1 Inhibitor, in Patients With Advanced Non–Small Cell Lung Cancer A Phase 1 Trial 2020 Open link
- 5 Cancer Communications A Phase I Study of Toripalimab, an anti-PD-1 Antibody, in Patients With Refractory Malignant Solid Tumors 2020 Open link
- 6 Clinical Cancer Research Safety, Efficacy and Biomarker Analysis of Toripalimab in previously treated advanced melanoma: results of the POLARIS-01 multicenter phase II trial 2020 Open link
- 7 European Journal of Cancer Safety and clinical efficacy of toripalimab, a PD-1 mAb, in patients with advanced or recurrent malignancies in a phase I study 2020 Open link
- 8 mABs Glycosylation-independent binding of monoclonal antibody toripalimab to FG loop of PD-1 for tumor immune checkpoint therapy 2019 Open link
- 9 Acta Pharmacologica Sinica Preclinical evaluation of the efficacy, pharmacokinetics and immunogenicity of JS-001 2017 Open link
- 10 Clinical Cancer Research Efficacy, safety and biomarkers of toripalimab in patients with recurrent or metastatic neuroendocrine neoplasms:a multiple-center phase Ib trial 2020 Open link
- 11 Journal of Clinical Oncology Axitinib in Combination With Toripalimab, a Humanized Immunoglobulin G4 Monoclonal Antibody Against Programmed Cell Death-1, in Patients With Metastatic Mucosal Melanoma: An Open-Label Phase IB Trial 2019 Open link
- 12 Annals of Translational Medicine JS001, an anti-PD-1 mAb for advanced triple negative breast cancer patients after multi-line systemic therapy in a phase I trial 2019 Open link
- 13 Annals of Oncology Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemorefractory gastric cancer treated with toripalimab, a PD1 antibody in phase Ib/II clinical trial NCT02915432 2019 Open link
- 14 Journal of Hematology & Oncology Safety and clinical activity with an anti-PD-1 antibody JS001 in advanced melanoma or urologic cancer patients 2019 Open link